Coronavirus
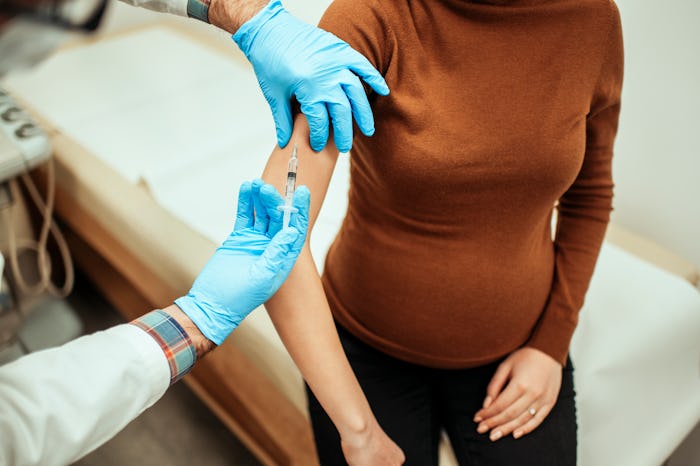
Pregnant Women Are Finally Included In COVID-19 Vaccine Trials
Pfizer and BioNTech are starting trials with 4,000 pregnant women.
As the Biden administration aims to vaccinate 100 million Americans during his first 100 days in office, researchers are now diving into how COVID-19 vaccines could specifically affect one vulnerable population. Pfizer and BioNTech announced this week via a joint statement that COVID-19 vaccine trials for pregnant people have begun and are happening around the globe.
More than 4,000 healthy pregnant women over the age of 18 and between 24 and 34 weeks gestation have signed on for the COVID-19 vaccine trials with Pfizer and BioNTech. According to a Feb. 18 press release, some will receive two doses of the BNT162b2 vaccine — also known as the Pfizer–BioNTech vaccine — while others will receive a placebo administered 21 days apart to determine the efficacy and safety of the product. Participants are expected to be in the trial for seven to 10 months.
Prior to the trial, Pfizer and BioNTech said studies were conducted on animals to ensure this trial would not affect subjects' fertility or reproductive toxicity. "Those studies showed no evidence of fertility or reproductive toxicity in animals," the press release stated.
"We are proud to start this study in pregnant women and continue to gather the evidence on safety and efficacy to potentially support the use of the vaccine by important subpopulations," Dr. William Gruber, Pfizer's senior vice president of vaccine clinical research and development, explained in a statement. "Pregnant women have an increased risk of complications and developing severe COVID-19, which is why it is critical that we develop a vaccine that is safe and effective for this population. We are deeply thankful to the volunteers who are enrolling in the trial, and site investigators who are leading this work."
Since COVID-19 first became a reality across the globe at the beginning of 2020, scientists and researchers have been attempting to get a sense of how this virus might affect pregnant people and those trying to get pregnant. While the elderly and chronically ill have been hit hardest by the spread of the coronavirus across the globe, the U.S. Centers for Disease Control and Prevention (CDC) notes that pregnant people are at an increased risk of becoming severely ill as well. COVID-19 could also lead to a higher chance of preterm birth, delivery via C-section, and infants being admitted into the NICU, as the Mayo Clinic explains.
Since Moderna, BioNTech, and Pfizer each found viable vaccines against COVID-19 late last year, millions of at-risk adults have been vaccinated. In the United States alone, more than 193 million doses of the vaccine have been administered to date, according to Bloomberg. The nation's top virologist Dr. Anthony Fauci noted recently, as Politico reported, that 20,000 pregnant people had so far been vaccinated in the United States "with no red flags." The CDC has also cleared pregnant people for getting the COVID-19 vaccine if they choose to do so.
Still, there had not yet been specific trials to investigate whether or not the vaccine would be safe for them until now. Historically speaking, there is a tendency to protect pregnant people from the potential risk of vaccine trials, but in this case the risk of contracting COVID-19 appears to be much more imperative. For expectant mothers as well as those planning to conceive.
"Enabling broad access to our highly effective COVID-19 vaccine is an important goal for us," Özlem Türeci, chief medical officer at BioNTech said in a statement, "Now that we are seeing successful initial implementation of vaccine campaigns with BNT162b2 across the globe, it is time to take the next step and extend our clinical program to other vulnerable populations, such as pregnant women, to potentially protect both them and future generations."
Trials will take place in the United States, Argentina, Brazil, Canada, Chile, Mozambique, South Africa, Spain and the United Kingdom, according to ABC News. Results are still a long way off, but it's a comfort to know that testing has begun.
If you think you’re showing symptoms of coronavirus, which include fever, shortness of breath, and cough, call your doctor before going to get tested. If you’re anxious about the virus’s spread in your community, visit the CDC for up-to-date information and resources, or seek out mental health support. You can find all of Romper’s parents + coronavirus coverage here.