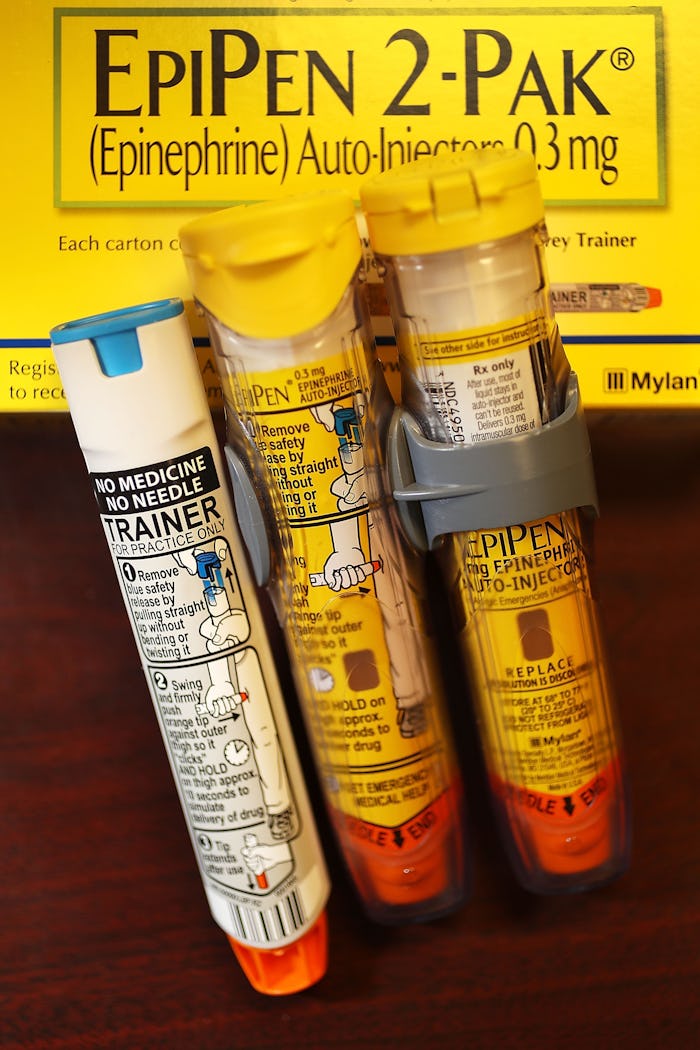
The Mylan company has just issued another recall. After recalling over 80,000 EpiPens worldwide recently, the company announced it had received two separate reports of potentially faulty devices outside the U.S.. Parents will naturally be wondering which Mylan EpiPens have been recalled, as this could be a serious health hazard to a child who relies on the lifesaving benefits of the EpiPen.
Romper has reached out to Mylan and is awaiting a response.
Mylan released a statement about the latest voluntary recall, according to Business Insider, which read:
This recall is being conducted as a result of the receipt of two previously disclosed reports outside of the U.S. of failure to activate the device due to a potential defect in a supplier component. The potential defect could make the device difficult to activate in an emergency (failure to activate or increased force needed to activate) and have significant health consequences for a patient experiencing a life-threatening allergic reaction (anaphylaxis).
The devices whuch have been included in the recall were made between Dec. 2015 and July 2016. The expiration dates of the devices are Apr. 2017, Sept. 2017, and Oct. 2017. The Mylan EpiPens, and EpiPen Jr., are made by Meridian Medical Technologies. This company also makes a Generic EpiPen that is not included in the recall, according to ABC 15.
UPI reported that the recalled EpiPen (0.3 mg) and EpiPen Jr. (0.15 mg) are sold as 2 Pak Auto-Injectors. The Mylan adult version are sold in North and South America, Asia, and Europe. They are equipped with a yellow label while the EpiPen Jr. is sold with a green label. According to the statement released by Mylan, the recalled products will be replaced at no extra cost to customers.
Mylan is committed to replacing recalled devices at no cost and Mylan would like to reassure patients that there will be no additional replacement-related financial burden to them as a result of this recall. Patients, customers and distributors are being notified and should refer to Mylan.com/EpiPenRecall for updates on product return and replacement instructions. We are asking patients to keep their existing product until their replacement product can be secured.
This is the second recall Mylan has announced in 10 days; the first was a worldwide recall of 80,000 EpiPens. These devices are used by patients who suffer from severe and potentially fatal allergic reations; EpiPens provide doses of epinephrine to patients with anaphylaxis.
Consumers with more questions can call Mylan Customer Relations at 800-796-9526 or email customer.service@mylan.com.